About us

AMS is a venture-backed Nuclear Medicine start-up leading the transformation of Japan’s nuclear sector. In the wake of the 2011 Fukushima nuclear accident, AMS is pioneering a new generation of nuclear medicine technologies that address systemic inefficiencies, regulatory bottlenecks, and limited access within the domestic market — through a dual focus on clinical innovation and infrastructure advancement. With two integrated business divisions — Theranostics Development (TD) and Engineering Solutions (ES) — AMS is positioned to shape the future of precision healthcare across the country.
In its TD business, AMS is introducing next-generation PET diagnostics, including 68Ga-PSMA, and supporting the development of novel radiotherapeutics such as astatine-211 (211At) to meet growing demand for targeted oncology solutions. These efforts are grounded in AMS’s proprietary expertise in medical device development, radiochemistry, and regulatory strategy. The resulting innovations are aligned with global trends and address pressing needs in a domestic market seeking scalable, high-impact care modalities.
Complementing this, AMS’s ES business tackles a critical barrier in the delivery of nuclear medicine: the safe and efficient management of radioactive discharge. The company has developed a proprietary bedside filtration system that streamlines regulatory compliance, reduces operational burden, and significantly improves patient throughput by enabling faster discharge and more rapid room turnover. This empowers hospitals to treat more patients within existing infrastructure, drives revenue growth, and facilitates earlier discharge for patients. Additionally, the platform generates actionable clinical data to support treatment decision-making and ensure the safe handling of radioactive materials.
With a differentiated portfolio, strong regulatory alignment, and robust investor backing, AMS presents a compelling opportunity in Japan’s revitalized nuclear medicine sector — where cutting-edge innovation converges with urgent healthcare needs and long-term market growth.
The AMS Growth Flywheel
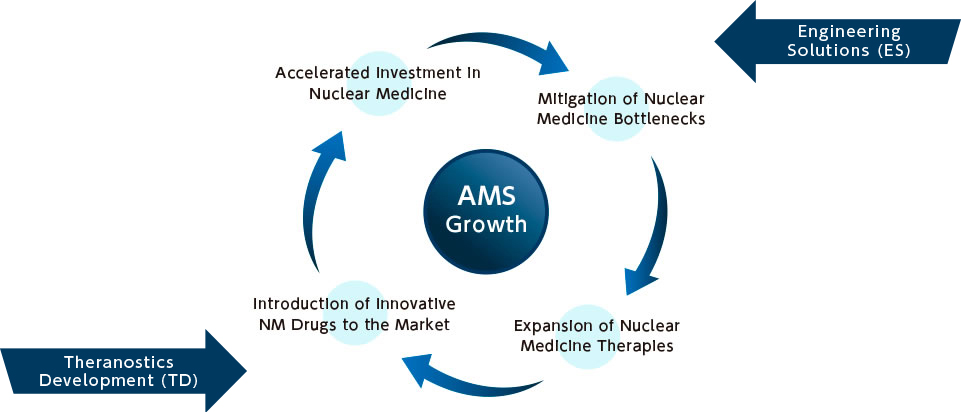
Our growth strategy is built on a four-stage self-reinforcing loop. At the core: AMS Growth, driven by the following pillars:
- 1.Mitigation of Bottlenecks in Nuclear Medicine
- We apply our platform capabilities to resolve infrastructure, regulatory, and operational constraints that limit the adoption of nuclear medicine.
- 2.Expansion of Nuclear Medicine Treatments
- Removing barriers enables increased clinical deployment, improving patient access and institutional readiness.
- 3.Introduction of Innovative Drugs to the Market
- We support the introduction and scaling of cutting-edge radiopharmaceuticals, bringing new therapeutic value to healthcare providers and patients.
- 4.Acceleration of Investment into the Sector
- As treatment volumes and drug pipelines grow, capital flows into the domain increase—supporting further expansion and reinforcing AMS’s leadership position.
Each element of the flywheel strengthens the others. Our Engineering Solution act as a catalyst for system-level transformation, while our Theranostics Platform Business anchors us in market impact and revenue generation.
Strategic Value for Investors
- ・Exponential Growth Potential
- The flywheel model is inherently scalable—each success accelerates the next phase of growth and amplifies shareholder value.
- ・Clear Market Positioning
- AMS is uniquely positioned to lead Japan’s nuclear medicine evolution, bridging systemic challenges with innovation and execution.
- ・Balanced Business Architecture
- Our dual-engine approach—combining platform innovation with therapeutic commercialization—creates defensibility and flexibility.
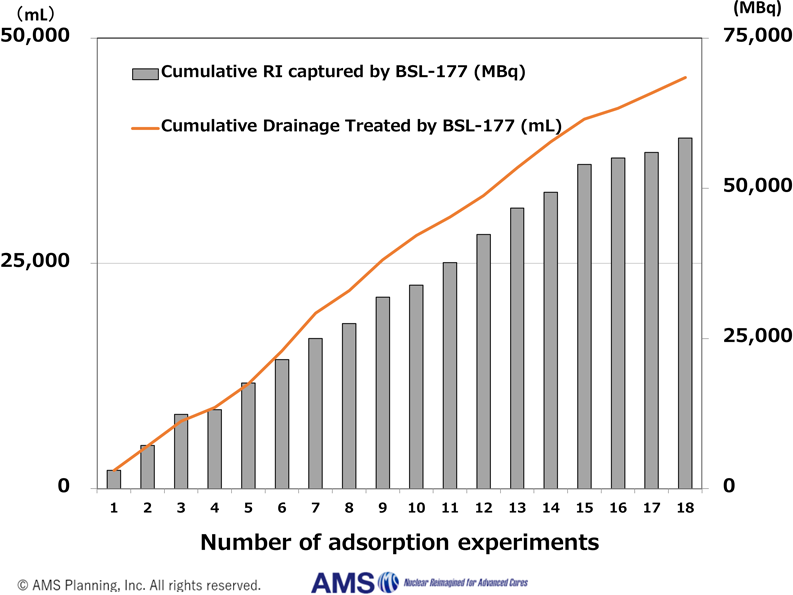
BSL-177 Performance as of Dec 2025
News
-
2026.2.24NEW2025年度 京都⼤学環境安全保健機構放射線管理部⾨共同利⽤成果発表会プログラムにおいて、弊社CEOが「RI医薬品の普及に伴う社会的課題と技術的解決〜BSXシステム開発から⾒える新たな研究領域〜(座長:京都⼤学環境安全保健機構放射線管理部⾨ ⽊村 寛之教授)」と題して、基調講演を行います。
-
2026.1.6NEW弊社の取り組みが、法人向けデジタルメディア「DOW JONES 読売新聞 Pro」に掲載されました。
「DOW JONES 読売新聞 Pro」の詳細については、こちらをご覧ください。 -
2025.12.24NEWBSL-177 has successfully captured over 60 GBq of radionuclides worldwide, marking a significant step toward demonstrating its robustness in clinical settings.
-
2025.12.15NEW国立研究開発法人 国立がん研究センター 中央病院において、共同研究(研究課題番号:2024-353、研究責任者:伊藤公輝先生)にもとづき、PSMA陽性転移性去勢抵抗性前立腺がん患者尿を対象にした177Lu吸着実験を開始します
-
2025.11.1NEWAMS、独ドレスデンのカール・グスタフ・カルス大学病院にBSL-177システムを設置 ― 日本国外で初の事例
AMS Installs BSL-177 at The Carl Gustav Carus Hospital in Dresden, Germany — First International Deployment Outside Japan -
2025.10.29NEW令和7年11月1日(土)開催の第98回 日本核医学会北日本地方会にて、北海道大学病院核医学診療科 渡邊史郎先生が、「Lu-177除去装置「BSL177」の使用経験」についてご発表されます。
-
2025.9.12NEWBSL-177 has successfully captured over 50,000 MBq of radionuclides, marking a significant step in demonstrating its robustness in clinical settings.
-
2025.8.14NEWAMS will participate in the UC Berkeley SkyDeck Entrepreneurship Bootcamp at Hokkaido University on August 20–21, 2025.
-
2025.8.7NEWAMSと東京パワーテクノロジー株式会社は、放射線利用の専門技術で日本をリードする株式会社千代田テクノルと共に、核種吸着システム「BSL-177」の医療現場への導入に向けた検討を開始しました。
-
2025.7.23NEW独Carl Gustav Carus University Hospitalを訪問し、Prof. Dr. med. Dr. rer. nat. Ralph A. Bundschuh(Director of Nuclear Medicine)とアクチニウム225吸着(BSA225)に関するディスカッションを行いました。
-
2025.5.26NEW第3回 J-KISS型新株予約権方式により資金調達を行いました。
-
2025.4.17NEW【AMS】2025年6月16日〜19日に米国ボストンで開催される「2025 BIO International Convention」ジャパンパビリオン出展企業に採択されました。
-
2025.1.14NEW資金調達を行いました。
-
2024.9.27NEW第2回 J-KISS型新株予約権方式により資金調達を行いました。
-
2024.5.29NEW第1回 J-KISS型新株予約権方式により資金調達を行いました。
-
2024.3.15NEW当社の合成装置ビジネスモデルに関するシステム特許が成立しました。(特許番号:特許第7455371号)
-
2024.1.22NEW放射性薬剤製造に関する特許を京都大学と共同出願しました。
-
2023.12.15NEWPET診断薬調製キット向け撹拌機に関する追加の特許を出願しました。
-
2023.11.1NEWAMS企画株式会社およびメディカルアイソトープ・プライムジャパン株式会社(MIPJ)は東京都港区白金台5丁目6番9号303号室にオフィスを移転いたしました。
-
2023.9.25NEW当社の68Ga標識PET診断薬ベースのセラノスティクスに関するシステム特許が成立しました。(特許番号:特許第7355370号)
-
2023.9.1NEWKeiichi Hirano was Appointed as the Chief Engineer of AMS.
-Hirano has 40 years of experience producing radiopharmaceuticals (GMP) and developing several types of Synthesizers to prepare At211-radiopharmaceuticals and others. -
2023.6.20NEWAMS will be at 2023 SNMMI Annual Meeting in Chicago, IL, USA
https://am.snmmi.org/iMIS/SNMMI-AM -
2023.5.10NEW内閣府原子力委員会「第16回原子力委員会定例会議」(令和5年5月9日(火))「医療用等ラジオアイソトープ製造・利用推進アクションプラン」進捗状況概要(PDFファイル)において、弊社が紹介されています(「6.68Ga‐PET製剤化の形態」(25ページ)に掲載)
-
2023.4.28NEW2023.5.20 AMS will be attending "第 36 回日本核医学会北海道地方会・第 14 回日本核医学技術学会北海道地方会・北海道 PET 研究会" hosted by JSNM Hokkaido, JSMNT Hokkaido and JSRT Hokkaido.
北海道 PET 研究会 16 時~17 時
特別講演
演題名:「PET/MRI、ソマトスタチン受容体イメージングで実践する腫瘍核医学」
講師:京都大学大学院医学研究科 放射線医学講座 助教 子安 翔 先生 -
2023.2.13NEWAMSは、2023年3月25日(土)から28日(火)まで北海道大学で開催される「日本薬学会第143年会(札幌)(主催 公益社団法人日本薬学会)」にて、光免疫療法・放射性薬品に関するランチョンセミナーを共催します
ランチョンセミナー LS04:3月26日(日)12:30~13:30
座長:久下 裕司先生(北大アイソトープ総合センター)
LS04-01 「放射性医薬品の開発から光免疫療法への展開」花岡 宏史先生(関西医科大学光免疫医学研究所)
LS04-02 「がんに対する新たな画像診断と治療“theranostics”」中本 裕士先生(京大院医) -
2023.2.13NEWAMS will be attending "第44回 獣医学学術交流基金群講演会「光と放射線を利用した新しいがん診断と治療技術」" hosted by Faculty of Veterinary Medicine, Hokkaido University.
-
2022.9.30NEWJETRO北米ライフサイエンス・ショーケース(アメリカ・ボストン)に参加し、弊社CEOがBio Labs Watertown/CIC Cambridgeでピッチを行いました。
CEO Made Pitches at Bio Labs Watertown and CIC Cambridge hosted by JETRO Hokkaido, Hokkaido University and Sapporo City. -
2022.9.11NEW第62回日本核医学会学術総会において、北大との共同研究について口頭発表しました。
Presentation of a collaborative research with Hokkaido University at the 62nd Annual Scientific Meeting of the Japanese Society of Nuclear Medicine. -
2022.8.4NEWメディカルアイソトープ・プライムジャパン株式会社は、米国よりラジオアイソトープ製品を輸入し、公益社団法人日本アイソトープ協会を通じたテスト販売を実施致しました。
MIPJ announce a successful trial supply of medical isotopes from the USA to clients in Japan via Japan Radioisotope Association. -
2022.3.16NEW高度管理医療機器等販売業の許可を取得しました。
-
2022.1.7NEWAdditional funding raised through a private placement of new shares
-
2021.12.6NEW弊社および弊社子会社は、今後取り扱うラジオアイソトープ製品について、公益社団法人日本アイソトープ協会を通じた販売を行うために、同製品の売買に係る製品供給基本契約を締結しました。
AMS and MIPJ Announce a Basic Supply Agreement with Japan Radioisotope Association, JRIA, to distribute radioisotopes through JRIA's network in compliance with Japanese laws. -
2021.12.1NEW弊社および弊社子会社は、放射性同位元素等の規制に関する法律第4条第1項の規定により放射性同位元素の販売業の届け出を行い、受理されました
AMS and MIPJ notified the Nuclear Regulation Authority, NRA, of the intention to engage in the importation, sale, and distribution of radioisotopes, pursuant to the provisions of the Cabinet Order, and were granted the permission by the NRA. -
2020.12.17NEWGranting Stock Options to recruit and retain experienced professionals.
-
2020.7.30NEW大江橋法律事務所パートナーである山口拓郎弁護士をお招きし「放射性医薬品市場における独占禁止法」をテーマにした社内勉強会を行いました。
-
2020.5.8NEWGranting Stock Options to recruit and retain experienced professionals.
-
2020.4.29NEWData sharing agreement between AMS and Klinikum rechts der Isar der Technischen Universität München (MRI) .
https://www.mri.tum.de/ -
2020.4.7NEWAMS to have filed a patent application regarding machine learning for distributed radiopharmaceutical production
-
2019.12.25NEWAdditional funding raised through a private placement of new shares
-
2019.9.10NEWAMS to have filed a patent application regarding Artificial Intelligence for theranostics
-
2019.7.23NEWAdditional funding raised through a private placement of new shares
-
2019.7.23NEWAppointment of new CEO and COO
Member
-
Yuichiro Sugawara, MBA (Representative Director, President and CEO)Suga founded AMS in 2019, motivated by minimally invasive therapy and Fukushima Daiichi Accident, which has led him to focus on nuclear medicine. Suga has 20 years of business development experience in TEPCO and other companies with various senior business management roles.
Suga has a bachelor’s and a master’s degree in engineering from Waseda University and an MBA from the Washington University in Saint Louis, Olin School of Business. -
Toshihide Kubo (Director and Chief Operating Officer)Kubo joined AMS in July 2019 as Director and Chief Operating Officer. Kubo has 30 years of strategic investment and business management experience at Marubeni corporation, including as an outside director of Given Imaging Ltd. (now Medtronic).
Kubo has a bachelor’s degree in engineering from Keio University. -
Hiroyuki Okada (Outside Director)Okada joined AMS as an outside director in December 2022. Okada joined TEPCO in 1996, where he was involved in the development of Next Generation Nuclear Reactors in the Nuclear Energy Division. He was then transferred to TNP Partners (VC), where he was in charge of hands-on support. With a deep understanding of "large enterprises and small business" and "business company and finance," he has accumulated much experience in strategic planning and implementation, from business composition to service and sales strategy.
Okada has a B.S. in Materials Science and an M.S. in Nuclear Engineering from the Tokyo Institute of Technology and holds a Chief Nuclear Reactor Engineer license in Japan. -
Komei Washino, Ph.D. (Chief Technology Officer)Washino joined AMS in Dec 2019 as Nuclear Medicine Technical Manager. Washino has 30 years of experience in drug development, research, and commercialization of innovative radiopharmaceuticals. Before AMS, Washino was a Director at Nihon Medi-Physics, held academic appointments as a research professor at Hokkaido University Graduate School of Medicine and visiting researcher at the National Institutes for Quantum and Radiological Science and Technology (QST).
Dr. Washino received his DVM, Doctor of Veterinary Medicine, and Ph.D. from the Faculty of Veterinary Medicine, Hokkaido University. -
Noriake Okamoto (Nuclear Medicine Academic Office Manager)Okamoto joined AMS in Feb 2019 as Nuclear Medicine Academic Office Manager. Okamoto has spent 40 years in commercial and business development roles in companies in the nuclear medicine sector, beginning his career with Daiichi-RI Pharma (Now Fujifilm Toyama Kagaku) in 1975, including as General Manager for Bristol-Myers Squibb and Dupont. Okamoto also held an academic appointment as a lecturer at CHIBA INSTITUTE OF SCIENCE, Faculty of Risk and Crisis Management, Department of Health and Medical Sciences.
Okamoto holds a pharmacist license in Japan, a bachelor of pharmacy from Shizuoka Prefectural college of Pharmacy and was a research fellow at Pharmaceutical Science, Tokyo University. -
Keiichi Hirano (Chief Engineer)Hirano joined AMS in Sep 2023 as Chief Engineer. Hirano has 40 years of experience in the production of radiopharmaceuticals (GMP), which has been distributed all over Japan in a safe, stable, and reliable manner for many years; the development of several types of Radiopharmaceutical Synthesizer for the production of radiopharmaceuticals labeled with Ga67/I123/F18 and more recently At211, where he has collaborated with significant universities and hospitals dedicated to nuclear medicine in Japan.
Before AMS, Hirano worked for Nihon Medi-Physics and its subsidiary.