Topics
Topic#3 Form of Preparation of 68Ga-Based PET-Radiopharmaceutical in Japan
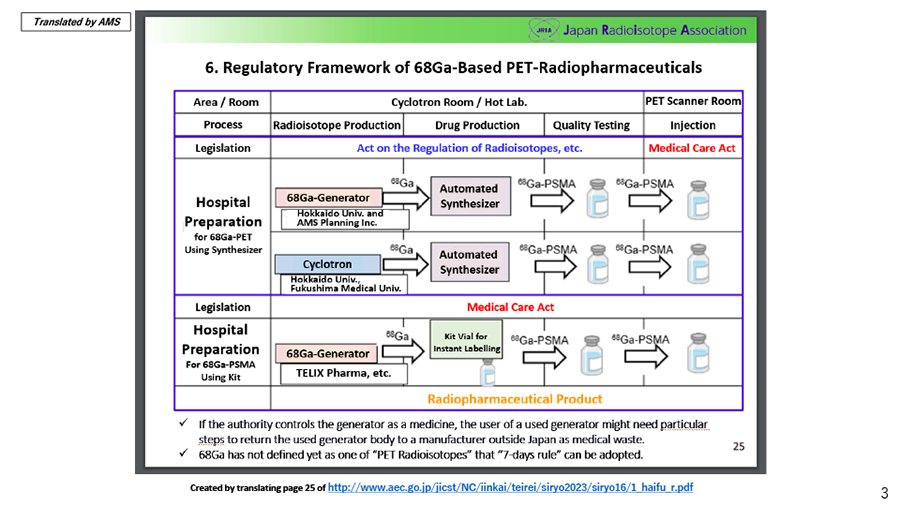
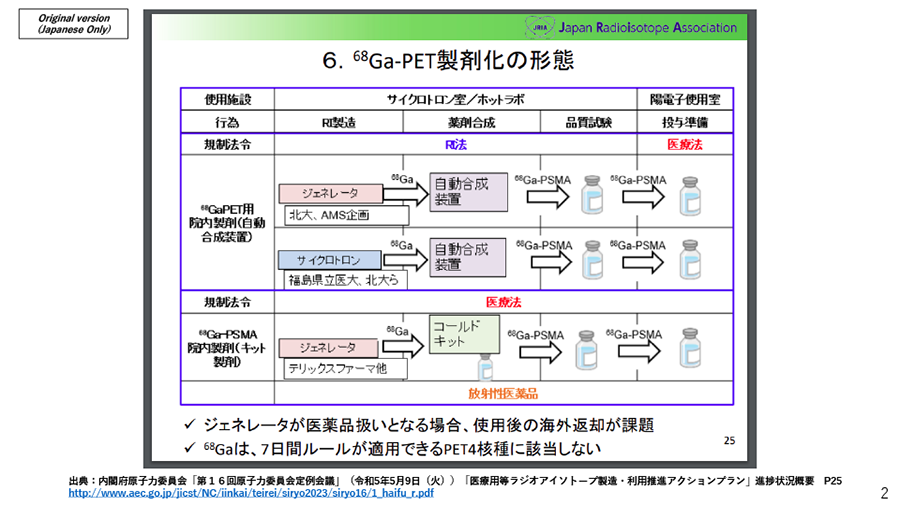
On May 9, 2023, The Japan Atomic Energy Commission (JAEC) published the materials on updating the overview of the plan for producing and utilizing medical isotopes, which The Japan Radioisotope Association (JRIA) prepared. In this document, AMS, together with Hokkaido University, was introduced as a case example that plans to combine a 68Ge/68Ga generator and a fully automated Synthesizer to prepare 68Ga-PSMA under the Act on the Regulation of Radioisotopes and the Medical Care Act.
Full version (Japanese only): http://www.aec.go.jp/jicst/NC/iinkai/teirei/siryo2023/siryo16/1_haifu_r.pdf
Full version (Japanese only): http://www.aec.go.jp/jicst/NC/iinkai/teirei/siryo2023/siryo16/1_haifu_r.pdf
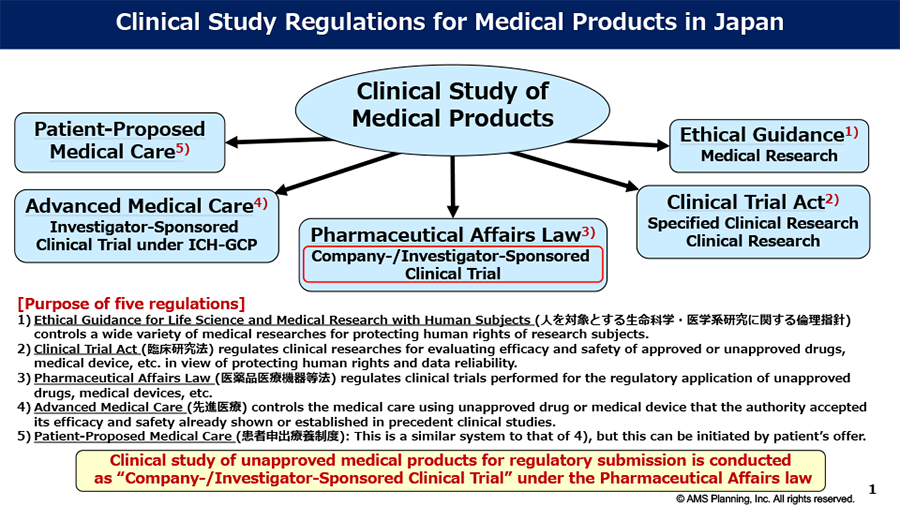
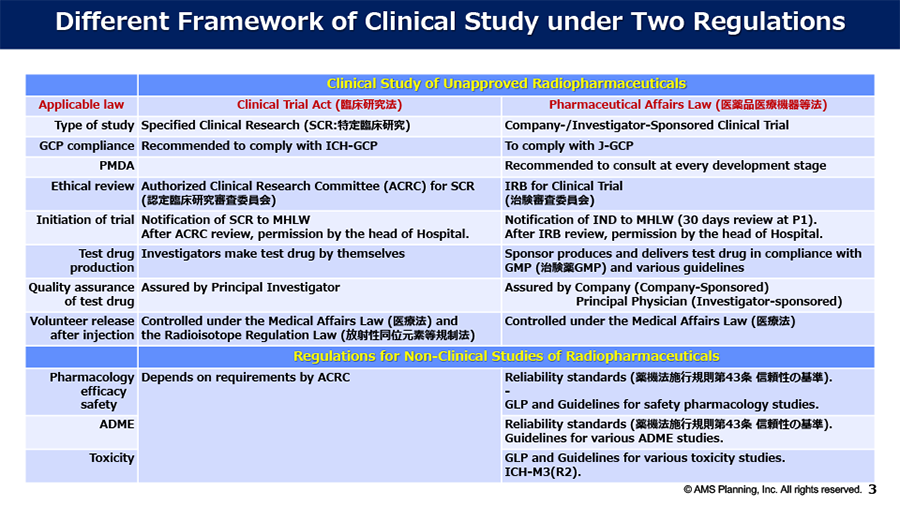
Topic#2 Regulatory Framework for Clinical Study of Radiopharmaceuticals in Japan
Since the international nuclear medicine players have frequently asked us about regulatory affairs regarding the Regulatory Framework for the Clinical Study of Radiopharmaceuticals in Japan, we would like to deliver our understanding for these issues as follows:
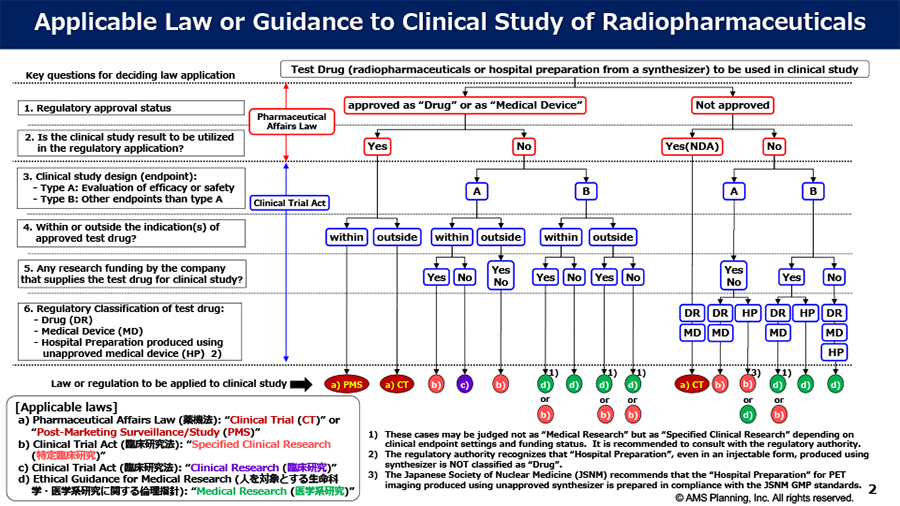
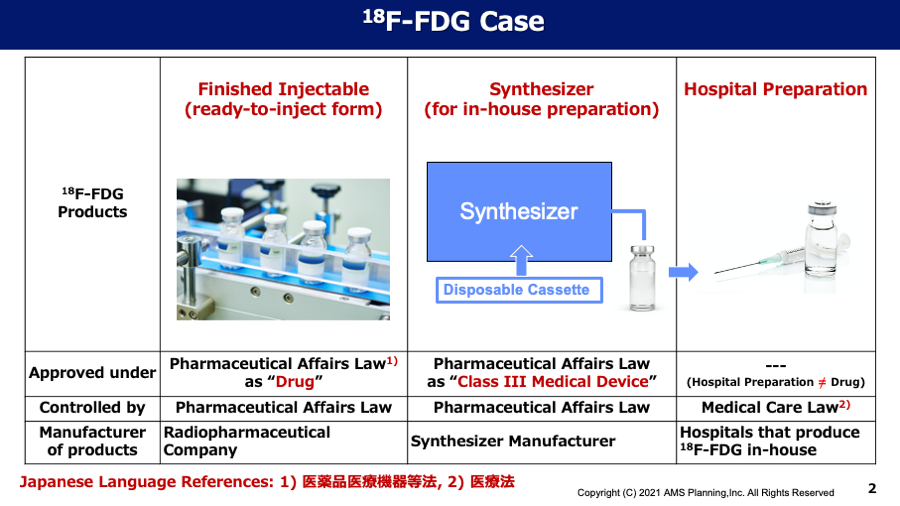
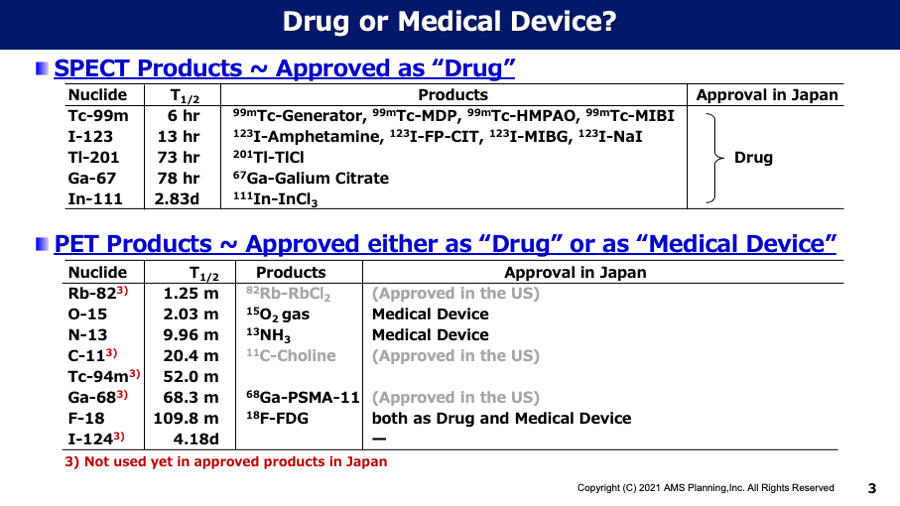
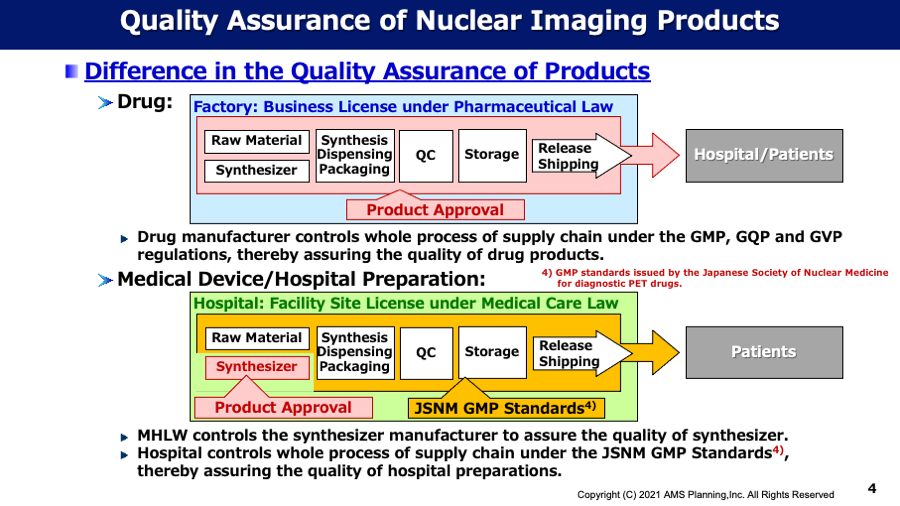
Topic#1 Medicine or Medical Device?
Since the international nuclear medicine players have frequently asked us about regulatory affairs regarding nuclear imaging products in Japan, especially PMDA application as medicine or medical device, we would like to deliver our understandings for these issues as follows: