Use of Sony’s Novel Material Triporous™ in the BSX System for Radioactive Urine Treatment in Nuclear Medicine
AMS and Tokyo Power Technology Ltd. (collectively, “the Companies”) announce the adoption of Triporous™, a novel material developed by Sony Group Corporation (“Sony”), in the BSX System, a liquid waste treatment system designed for radioactive urine management in nuclear medicine.
Triporous is utilized in the BSX System specifically for the adsorption of ammonia gas generated during system operation.


■Background
In nuclear medicine, urine excreted after radiopharmaceutical therapy may contain not only radioactive nuclides but also volatile components such as ammonia gas originating from urine.
From the perspective of occupational safety and comfort within controlled radiation areas, appropriate countermeasures for these gaseous components are required.
The BSX System was developed to address such challenges specific to nuclear medicine practice. In its development, the Companies have conducted material selection with careful consideration of gas adsorption performance, stability, and operational suitability.
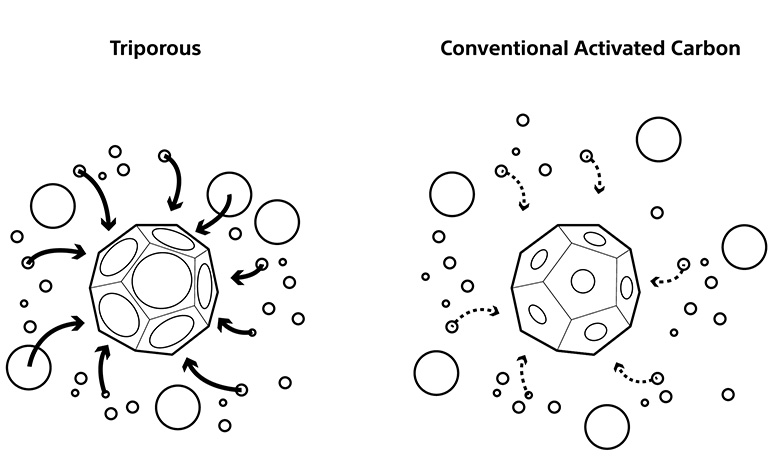
■About Triporous
Triporous is a porous carbon material derived from rice husks, independently developed by Sony.
It features a unique micro-porous structure and exhibits adsorption properties for various substances such as gases and odor-causing substances, leading to its application across a range of environmental fields.
Key characteristics of Triporous include:
- Use of naturally derived, biomass-based raw materials, contributing to sustainability
- Adsorption properties enabled by fine and diverse pore structures
- Compatibility with other materials, allowing flexible system design
■ Role within the BSX System
Within the BSX System, Triporous is applied as part of a material design strategy to address volatile components such as ammonia gas generated during radioactive urine treatment.
By leveraging the material’s characteristics and optimizing material configurations according to operational conditions, the system aims to ensure overall adsorption performance and operational stability.
This approach is intended to:
- Reduce ammonia gas and other volatile components generated during radioactive urine treatment
- Improve odor control and working environment comfort
- Support stable operation in nuclear medicine facilities
■Future Outlook
Building on materials science and environmental technology expertise accumulated in Japan, the Companies continue to advance the development of nuclear medicine infrastructure with global competitiveness.
In the ongoing development of the BSX System, the Companies are also engaged in the systematic accumulation of knowledge regarding the chemical characteristics of post-therapy urine, including the properties of radiopharmaceutical metabolites and the proportion of unchanged compounds.
These insights are being incorporated into material selection and system configuration studies.
Through technology development closely aligned with clinical practice, the Companies remain committed to contributing to the enhanced safety, reliability, and sustainability of infrastructure supporting nuclear medicine therapies.


